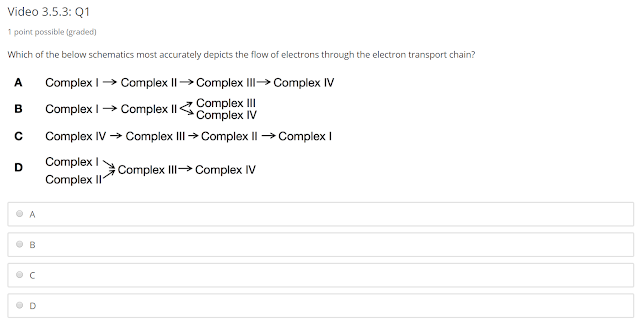
The correct answer is D.
Complex I and Complex II takes electrons from NADH and FADH2 respectively and transfer them to Coenzyme Q. Complex III take electrons from reduced Coenzyme Q to cytochrome C. Finally, Complex IV takes electron reduced cytochrome C to oxygen.
NADPH - this molecule is not involved in the electron transport chain.
$ O_2 $
Protons are transmitted across the mitochondrial membrane by the ETC, building up a gradient which is used to drive ATP synthesis.
To transfer a single electron to cytochrome c from the double electron carrier QH2.
To transfer electrons from universal electron acceptors to coenzyme Q.
Here are the equations for the electron transfer steps:
NADH + 5H(m) + Q -> NAD+ + QH(2) + 4H(i)
QH(2) + 2CYTC(ox) + 2H(m) -> Q + 2CYTC(red) + 4H(i)
4CYTC(red) + 8H(m) + O2 -> 4CYTC(ox) + 2H2O + 4H(i)
Taking the double of the first two equations, we get:
2NADH + 10H(m) + 2Q -> 2NAD+ + 2QH(2) + 8H(i)
2QH(2) + 4CYTC(ox) + 4H(m) -> 2Q + 4CYTC(red) + 8H(i)
4CYTC(red) + 8H(m) + O2 -> 4CYTC(ox) + 2H2O + 4H(i)
And then sum them up, we get
2NADH + 10H(m) + 2Q + 2QH(2) + 4CYTC(ox) + 4H(m) + 4CYTC(red) + 8H(m) + O2
->
2NAD+ + 2QH(2) + 8H(i) + 2Q + 4CYTC(red) + 8H(i) + 4CYTC(ox) + 2H2O + 4H(i)
Therefore the answers are:
20 protons
2 molecules of water, and
1 molecule of oxygen
The number of protons worth a little more discussion here, obviously, we consumed 22 free protons in the matrix and produced 20 free protons in the intermembrane space. We count that as pumped 20 protons, we could as well say we pumped 22. The two protons, together with the two disassociated from the NADH, are used to form water.






No comments:
Post a Comment