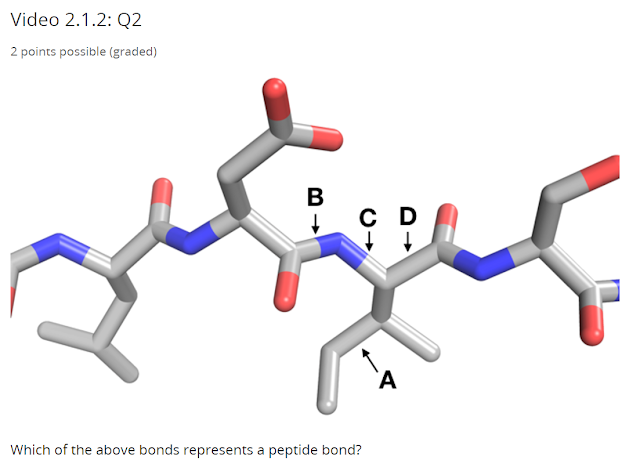
The answer for the first part is (A) - this is the carboxyl carbon - it has a single bond to the alpha carbon, a single bond to the amine nitrogen, and a double bond to an oxygen, the double bond is what make it $ sp^2 $ hybridized.
The answer for the second part is (C) - this is the alpha carbon, the bond to hydrogen is implicit and is not drawn.
The answer for the third part is (A) and (B) - this is the peptide bond, the bonding between the carboxyl carbon and the amine nitrogen.
Just like the above, the peptide bond is the bond between the carboxyl carbon and the amine nitrogen, therefore the answer is (B)
Therefore the only remaining two bonds are (C) and (D) and they are the answer.
If it is all green, it should be an alpha helix.
The blue shared area can form beta sheets.
All areas can form loops.
Therefore, the only possible answer is the last choice, the most common combinations of bond angles in proteins.
It only make sense to group "like" things together, on the right hand side, we see some polar looking stuff with blue and red, therefore, it only make sense if the left hand side is non-polar. The side chains are pointing outwards, they can't help stabilizing the alpha helix (unless they clash into each other - in which they destabilize it) - so the first choice is wrong. The third choice is hard to tell, but for obvious reason the drawing included a short side chain, so that isn't true either. Non-polar stuff do not form hydrogen bond, so that's false as well. The only reasonable choice is the second one, so that's the answer.
As a side note - I have no idea why the answer stress that that side chains are aliphatic, I think it should be just fine even if the side chains contains a benzene ring, as long as it stays non-polar.
I did some searching on this one. Most amino acids has the trans configuration. That is to minimize steric hindrance. This implies the third choice is wrong. Flipping between cis and trans configuration for proline is known to be slow (and is often the rate determining step), so the fourth choice is wrong as well. While the second choice is unclear, the first one is definitely true, attached below we can see the proline structure, the usually exposed free hydrogen bonded with nitrogen is now participating in a ring, so it can't use that free hydrogen to hydrogen bond with the carboxylate oxygen to stabilize the helix. That's why the answer is the first choice.











No comments:
Post a Comment