To put it simply, two different 3D structures are called conformations of each other if a molecule can transform from one to another without having to break a bond. Otherwise it is a configuration change.
Therefore, the first answer is:
"The (potentially flexible) spatial arrangement of atom around fixed bonds in a molecule"
And the second answer is:
"The fixed arrangement of atoms dictated by bonds of a molecule"
It is also chiral, and it has a lot of chiral centers
This is a little tricky - the answer is no. The drawings are exactly the same thing.

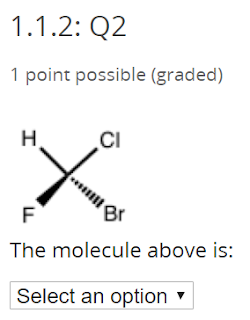


No comments:
Post a Comment